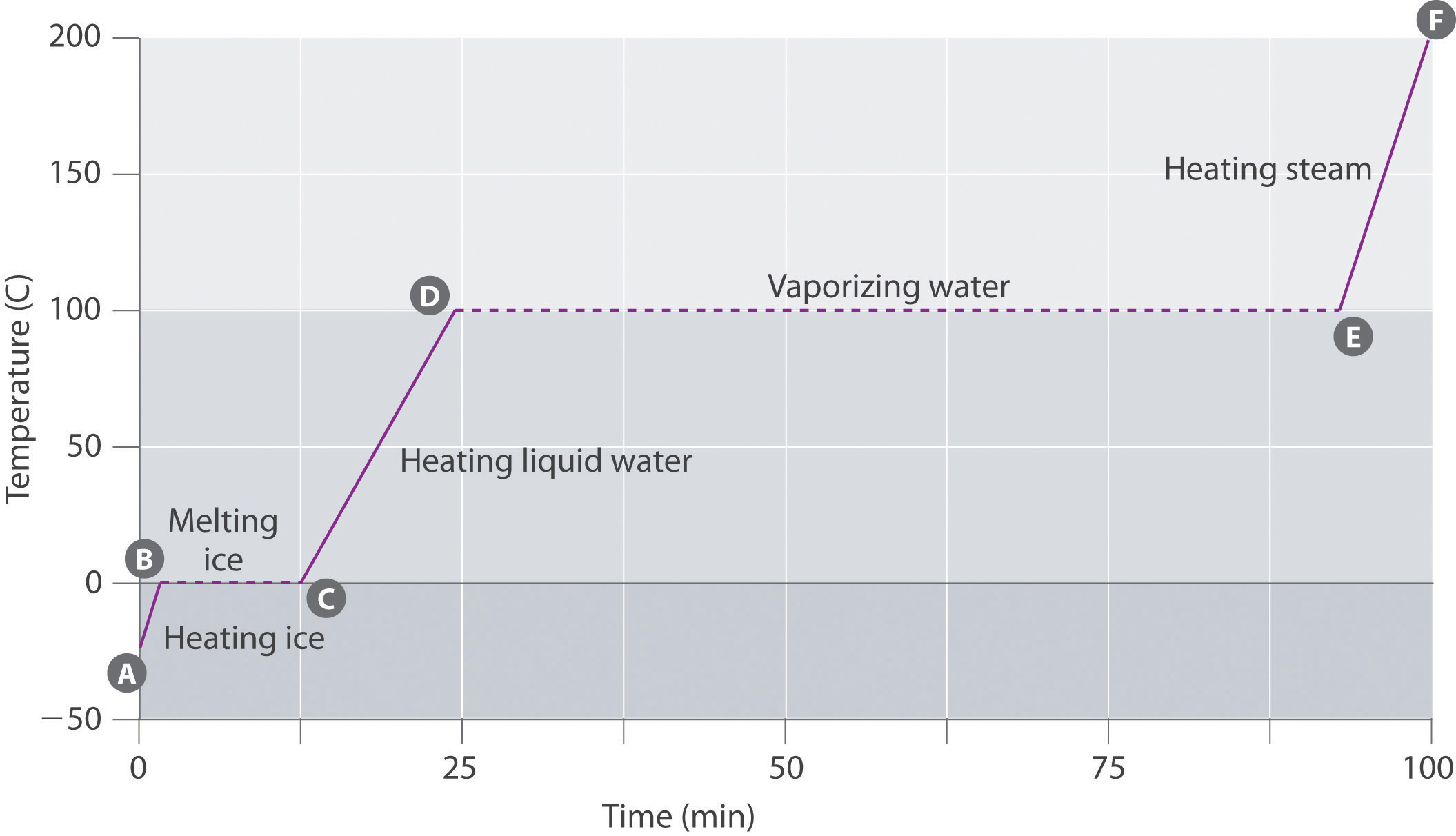
Heating Curve Of Water Table . When a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Now let's look at a schematic diagram of continuous heating of water from a. Since temperature is a measure of average kinetic energy, any change in temperature is a change in kinetic energy. The sample is initially ice at 1. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The heating curve of water. The ice is in a closed. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The heating curve for water shows how the temperature of a given quantity of water changes.
from chem.libretexts.org
The ice is in a closed. Since temperature is a measure of average kinetic energy, any change in temperature is a change in kinetic energy. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1. The heating curve for water shows how the temperature of a given quantity of water changes. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. When a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and. The heating curve of water. Now let's look at a schematic diagram of continuous heating of water from a.
11.7 Heating Curve for Water Chemistry LibreTexts
Heating Curve Of Water Table The heating curve of water. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. When a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and. Since temperature is a measure of average kinetic energy, any change in temperature is a change in kinetic energy. Now let's look at a schematic diagram of continuous heating of water from a. The heating curve for water shows how the temperature of a given quantity of water changes. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The ice is in a closed. The heating curve of water.
From www.chegg.com
Solved 12. This diagram shows a heating curve for ice Heating Curve Of Water Table The heating curve of water. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The sample is initially ice at 1. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Figure 11.4.1 shows a. Heating Curve Of Water Table.
From preparatorychemistry.com
Heating Curve Heating Curve Of Water Table The heating curve of water. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The ice is in a closed. The heating curve for water. Heating Curve Of Water Table.
From studylib.net
Heating Curve Lab Heating Curve Of Water Table Now let's look at a schematic diagram of continuous heating of water from a. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The heating. Heating Curve Of Water Table.
From www.animalia-life.club
A Heating Curve Illustrates Heating Curve Of Water Table The heating curve for water shows how the temperature of a given quantity of water changes. The sample is initially ice at 1. Since temperature is a measure of average kinetic energy, any change in temperature is a change in kinetic energy. The heating curve of water. The ice is in a closed. Imagine that you have a block of. Heating Curve Of Water Table.
From quizlet.com
Heating Curve for Water Diagram Quizlet Heating Curve Of Water Table Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The heating curve for water shows how the temperature of a given quantity of water changes. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point.. Heating Curve Of Water Table.
From allisonrswankxo.blob.core.windows.net
Heating Cooling Curve Of Water Heating Curve Of Water Table Since temperature is a measure of average kinetic energy, any change in temperature is a change in kinetic energy. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Now let's look at a schematic diagram of continuous heating of water from a. Imagine that you have a block of. Heating Curve Of Water Table.
From ar.inspiredpencil.com
Specific Heat Of Water Chart Heating Curve Of Water Table The ice is in a closed. When a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The sample is initially ice at 1. Now let's look at a schematic diagram of continuous heating of water. Heating Curve Of Water Table.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Of Water Table Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Now let's look at a schematic diagram of continuous heating of water from a. The heating. Heating Curve Of Water Table.
From scienceisntscary.wordpress.com
Boiling point Ease Into Science Heating Curve Of Water Table The heating curve of water. When a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The ice is in a closed. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for. Heating Curve Of Water Table.
From slideplayer.com
Chapter 1 Matter and Change. ppt download Heating Curve Of Water Table The heating curve of water. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Now let's look at a schematic diagram of continuous heating of water from a. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. When. Heating Curve Of Water Table.
From www.researchgate.net
Heating curve of water at fixed atmospheric pressure. It corresponds to Heating Curve Of Water Table Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Now let's look at a schematic diagram of continuous heating of water from a. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The sample. Heating Curve Of Water Table.
From studylib.net
Heating Curve of Water Activity Heating Curve Of Water Table The ice is in a closed. Now let's look at a schematic diagram of continuous heating of water from a. Since temperature is a measure of average kinetic energy, any change in temperature is a change in kinetic energy. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. When. Heating Curve Of Water Table.
From lessonzoneyvonne.z13.web.core.windows.net
Heating Curve Of Water Worksheet Heating Curve Of Water Table The sample is initially ice at 1. Since temperature is a measure of average kinetic energy, any change in temperature is a change in kinetic energy. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Heating Curve Of Water Table.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation Heating Curve Of Water Table The ice is in a closed. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The heating curve for water shows how the temperature of a given quantity of water changes. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for. Heating Curve Of Water Table.
From www.chegg.com
Solved Using The Data Below, Create A Graph To Demonstrat... Heating Curve Of Water Table Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The ice is in a closed. The heating curve of water. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Imagine that you have a block of ice that. Heating Curve Of Water Table.
From www.youtube.com
Heating Curve of Water YouTube Heating Curve Of Water Table Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The heating curve for water shows how the temperature of a given quantity of water changes.. Heating Curve Of Water Table.
From quizzmediakrueger.z13.web.core.windows.net
Worksheets Heating Curve Of Water Heating Curve Of Water Table The sample is initially ice at 1. The heating curve of water. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The ice is in a closed. Now let's. Heating Curve Of Water Table.
From www.chegg.com
Solved d. Examine the heating curve for water below. Answer Heating Curve Of Water Table The sample is initially ice at 1. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. When a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and. The ice is in a closed. The heating curve of water. Figure \(\pageindex{3}\) shows a heating curve, a. Heating Curve Of Water Table.